Living systematic reviews (LSRs) are a new approach to conducting systematic reviews that allow for real-time updates to be made as new evidence becomes available.
A recent study published in BMJ Evidence-Based Medicine highlights the benefits and challenges of LSRs and emphasizes the importance of adopting this approach in healthcare research.
LSRs differ from traditional systematic reviews in that they are continuously updated to reflect the latest research. This allows for rapid dissemination of new findings and ensures that the review remains current and relevant over time. LSRs are particularly useful in areas of research that are rapidly evolving or where new evidence is frequently emerging.
The study highlights the potential benefits of LSRs, such as their ability to provide timely and relevant evidence to inform clinical decision-making, and their potential to reduce research waste and duplication. However, the study also notes that there are challenges to implementing LSRs, including the need for clear protocols, robust search strategies, and effective management of data and evidence.
Despite these challenges, the authors of the study argue that LSRs have the potential to revolutionize the way we conduct systematic reviews and generate evidence for healthcare decision-making. The use of LSRs could lead to more efficient and effective research, as well as better outcomes for patients.
As the field of healthcare research continues to evolve, it is likely that we will see an increasing adoption of LSRs. These living reviews have the potential to transform the way we approach evidence-based medicine and provide new opportunities for improving patient care.
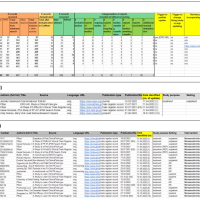
“Living systematic reviews (LSRs) are an increasingly common approach to keeping reviews up to date, in which new relevant studies are incorporated as they become available, so as to inform healthcare policy and practice in a timely manner. While journal publishers have been exploring the publication of LSRs using different updating and publishing approaches, readers cannot currently assess if the evidence underpinning a published LSR is up to date, as neither the search details, the selection process, nor the list of identified studies is made available between the publication of updates. We describe a new method to transparently report the living evidence surveillance process that occurs between published LSR versions. We use the example of the living Cochrane Review on nirmatrelvir combined with ritonavir (Paxlovid) for preventing and treating COVID-19 to illustrate how this can work in practice. We created a publicly accessible spreadsheet on the Open Science Framework platform *”. – say the methodologist Stephanie Weibel** (Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Würzburg) and the information specialist Maria-Inti Metzendorf (Institute of General Practice, Medical Faculty of the Heinrich-Heine University, Heinrich Heine University Düsseldorf), who developed the LSR approach together with their team during the COVID-19 pandemic.
Link to the publication in BMJ Evidence-Based Medicine: Metzendorf MI, Weibel S, Reis S, McDonald S. Pragmatic and open science-based solution to a current problem in the reporting of living systematic reviews. BMJ Evid Based Med. 2022 Nov 9:bmjebm-2022-112019. doi: 10.1136/bmjebm-2022-112019.
*The spreadsheet shows three of the four tabs available in the public OSF table (https://osf.io/7g49c/ ). Review readers can easily access the Cochrane review's new underlying evidence via the OSF link, to find out if new studies have been identified that could affect the results, to track ongoing studies and to track the progress of studies through the review. Further information is available at http://dx.doi.org/10.1136/bmjebm-2022-112019
**Stephanie Weibel is an Editorial Board member of the Denmark-based CRGs, Cochrane CARG and EC. She is also part of the https://covid-nma.com/ operating team.
